Abstract
Responses to FLT3-inhibitors are usually transient due to emergence of resistance through the acquisition of kinase domain point mutations and other non-mutational mechanisms. SEL is a potent first-in-class Selective Inhibitor of Nuclear Export/SINE™ that exerted marked cell killing of human and murine FLT3-mutant AML cells, including those with ITD, D835Y, ITD+Y842C or ITD+F691L mutations by modulating the cdk inhibitor p27 and anti-apoptotic Mcl-1. The combination of SEL+sorafenib had synergistic pro-apoptotic effects in FLT3-mutated AML cells by suppressing phosphorylation levels of FLT3 and its downstream signaling mediators ERK/AKT, and by inducing myeloid differentiation in ITD and D835 mutated cell lines.
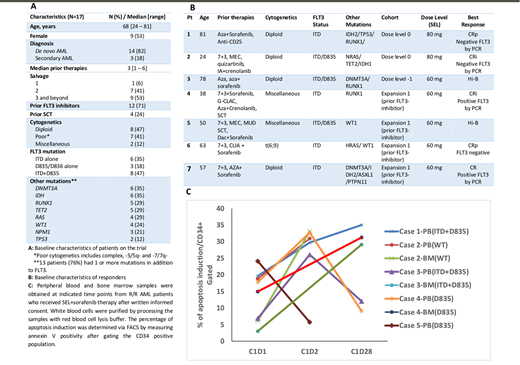
We designed a phase I/II trial of SEL with sorafenib for FLT3-ITD and/or -D835 R/R AML pts. In phase I, the primary objectives were to determine the maximum tolerated dose (MTD), the recommended phase 2 dose (RP2D), and dose-limiting toxicity (DLT) of the combination. In phase II, primary objectives included the rate of composite complete remission (CRc) defined as CR+CR with incomplete platelet recovery (CRp)+CR with incomplete count recovery (CRi) within 3 months (mos) of therapy initiation. Secondary objectives were safety and overall survival (OS). SEL was given orally twice a week for 3-weeks on and 1-week off in 28-day cycles and sorafenib was given continuously at a dose of 400mg twice daily from cycle 1 day 1 of SEL. In phase I, SEL dose was de-escalated in "3+3" fashion starting at dose level 0 and going down if DLTs were exceeded (dose levels 0, -1, -2 = 80, 60, 40 mg, respectively). Once MTD was established, the phase II enrolled pts in 2 cohorts: prior FLT3-inhibitor failure (cohort 1) and FLT3-inhibitor naïve (cohort 2). PB or BM samples were collected at pre-dose (C1D1), 24 h (C1D2) and day 28 (C1D28) of cycle 1, and apoptosis induction was determined by measuring annexin V positivity with FACS.
17 pts with median (med) age of 68 yrs (range 24-81) were enrolled. All pts had baseline next generation sequencing for AML specific mutations (Fig 1A). Median number of prior therapies was 3 (range, 1-6) as follows: salvage (S) 1: n=1, S2: n=7, S3+: n=9. 12 (71%) pts had prior FLT3-inhibitor exposure: 1 prior FLT3-inhibitor (n=10); 2 prior FLT3-inhibitors: n= (2). Four pts had prior allogeneic stem cell transplant.
Four pts were treated at dose level 0 (SEL 80mg) with DLTs in 2 (Grade (G) 3 sepsis, n=1; G3 mucositis, syncope, adrenal insufficiency, n=1). Three pts were subsequently treated at dose level -1 (SEL 60mg) with no DLTs and this was established as the MTD/RP2D. Ten pts were treated in expansion: 7 had prior FLT3-inhibitor therapy (cohort 1) and 3 had no prior FLT3-inhibitor (cohort 2). Med duration of treatment for all 17 pts was 55 days (range 14-122) and all pts are off-protocol. All pts are evaluable for response. Overall, 5 pts (29%) achieved a CRc including CR (1/17; 6%), CRi (2/17, (12%) CRp(2/17; 12%). Interestingly, all responders had prior FLT3 inhibitor exposure (1 prior FLT3i in 3 pts, 2 prior FLT3i in 2 pts) indicating a CRc rate of 45% (5/11) in pts with prior FLT3 inhibitor exposure. Two pts with CRi and 1 with CRp (2 with FLT3-ITD and 1 with ITD+D835) achieved negative RT-PCR for FLT3 at time of response (Fig 1B). Med DOR was 2.6 mos (range 1-6.1). Med OS for all pts on study was 4 mos (range 0.9-16.54) and 16.54 mos for responders (CR/CRi/CRp) (range 2.7-16.54). One pt in CRi was bridged to SCT and remained alive at 1.5 yrs. G 1/2 adverse events (AE) included anorexia (n=8), nausea/vomiting (n=8) and diarrhea (n=4). G 3/4 AE irrespective of causality were bleeding (gastrointestinal n=4; intracranial n=1), febrile neutropenia (n=5), pneumonia (n=3), syncope (n=2), hyponatremia (n=2), weakness (n=1) and fatigue (n=1). Drug related G 3/4 AE were hyponatremia (n=2), mucositis (n=1), adrenal insufficiency (n=1), and fatigue (n=1). The reasons for discontinuing study therapy were disease progression (n=13; 76%), toxicity (n=3; 18% including fatigue, mucositis and sepsis), SCT (n=1; 6%). On translational analysis, SEL+sorafenib induced apoptosis in 4/5 tested pts, especially in BM samples (Fig 1C).
The combination of SEL+sorafenib was safe and clinically active with apoptosis induction in heavily pre-treated R/R FLT3+ AML. The benefit was especially encouraging in pts exposed to prior FLT3 inhibitor, with 45% CR/CRi/CRp rate (5/11; including 1 CR, 2 CRi and 2 CRp). The RP2D is 60 mg twice weekly of SEL
Daver:Otsuka: Consultancy; Pfizer: Research Funding; ARIAD: Research Funding; Novartis: Research Funding; Kiromic: Research Funding; Incyte: Research Funding; Alexion: Consultancy; Sunesis: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Karyopharm: Research Funding; Sunesis: Research Funding; Daiichi-Sankyo: Research Funding; BMS: Research Funding; Karyopharm: Consultancy; ImmunoGen: Consultancy; Incyte: Consultancy. Cortes:Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Arog: Research Funding. Ravandi:Seattle Genetics: Research Funding; Xencor: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Abbvie: Research Funding; Macrogenix: Honoraria, Research Funding; Seattle Genetics: Research Funding; Jazz: Honoraria; Orsenix: Honoraria; Sunesis: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Sunesis: Honoraria; Xencor: Research Funding; Bristol-Myers Squibb: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Astellas Pharmaceuticals: Consultancy, Honoraria; Abbvie: Research Funding; Jazz: Honoraria; Bristol-Myers Squibb: Research Funding; Macrogenix: Honoraria, Research Funding; Orsenix: Honoraria. Konopleva:Stemline Therapeutics: Research Funding. Kadia:Pfizer: Consultancy, Research Funding; Takeda: Consultancy; Jazz: Consultancy, Research Funding; Novartis: Consultancy; BMS: Research Funding; Takeda: Consultancy; Amgen: Consultancy, Research Funding; Novartis: Consultancy; Abbvie: Consultancy; Pfizer: Consultancy, Research Funding; Jazz: Consultancy, Research Funding; BMS: Research Funding; Abbvie: Consultancy; Celgene: Research Funding; Amgen: Consultancy, Research Funding; Celgene: Research Funding. Jabbour:Abbvie: Research Funding; Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Novartis: Research Funding. DiNardo:Bayer: Honoraria; Medimmune: Honoraria; Celgene: Honoraria; Agios: Consultancy; Abbvie: Honoraria; Karyopharm: Honoraria. Andreeff:Astra Zeneca: Research Funding; SentiBio: Equity Ownership; Jazz Pharma: Consultancy; Aptose: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Amgen: Consultancy, Research Funding; Oncolyze: Equity Ownership; Daiichi-Sankyo: Consultancy, Patents & Royalties: MDM2 inhibitor activity patent, Research Funding; United Therapeutics: Patents & Royalties: GD2 inhibition in breast cancer ; Reata: Equity Ownership; Oncoceutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Eutropics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal